PhD research: Thermodynamic assessment of the B-C-Hf-Zr system
This summarises the research contained within the PhD thesis, Thermodynamic assessments of the (Zr, Hf) carbides and borides revisited and informed by the calculation of defect formation energies in ZrC [1].
Review of the B-C-Hf-Zr system
The boron-carbon-hafnium-zirconium system contains several ultra-high-temperature ceramic (UHTC) compounds of use in the aerospace and nuclear industries thanks to their extremely high melting points and strength and hardness at high temperature. The phase diagrams and related literature describing this system was thoroughly reviewed and key potential improvements identified. Ab initio density functional theory (DFT) calculations were performed to complement the literature [1].
Incorporating the vacancy formation energy into the CALPHAD approach
Developments in first-principles calculations have recently allowed access to properties such as vacancy formation energies with uncertainties comparable with experiments. Although such quantities are implicitly described with the CALPHAD approach, they have not conventionally been considered directly. By rearranging terms in the parameterisation of the Gibbs energy, first-principles calculations of the vacancy formation energy in ZrC were compared with the CALPHAD assessments. A method was developed whereby such energies may be directly incorporated into the thermodynamic description either as part of the parameter optimisation or post hoc [1]. You can read more about continuations of this research here.
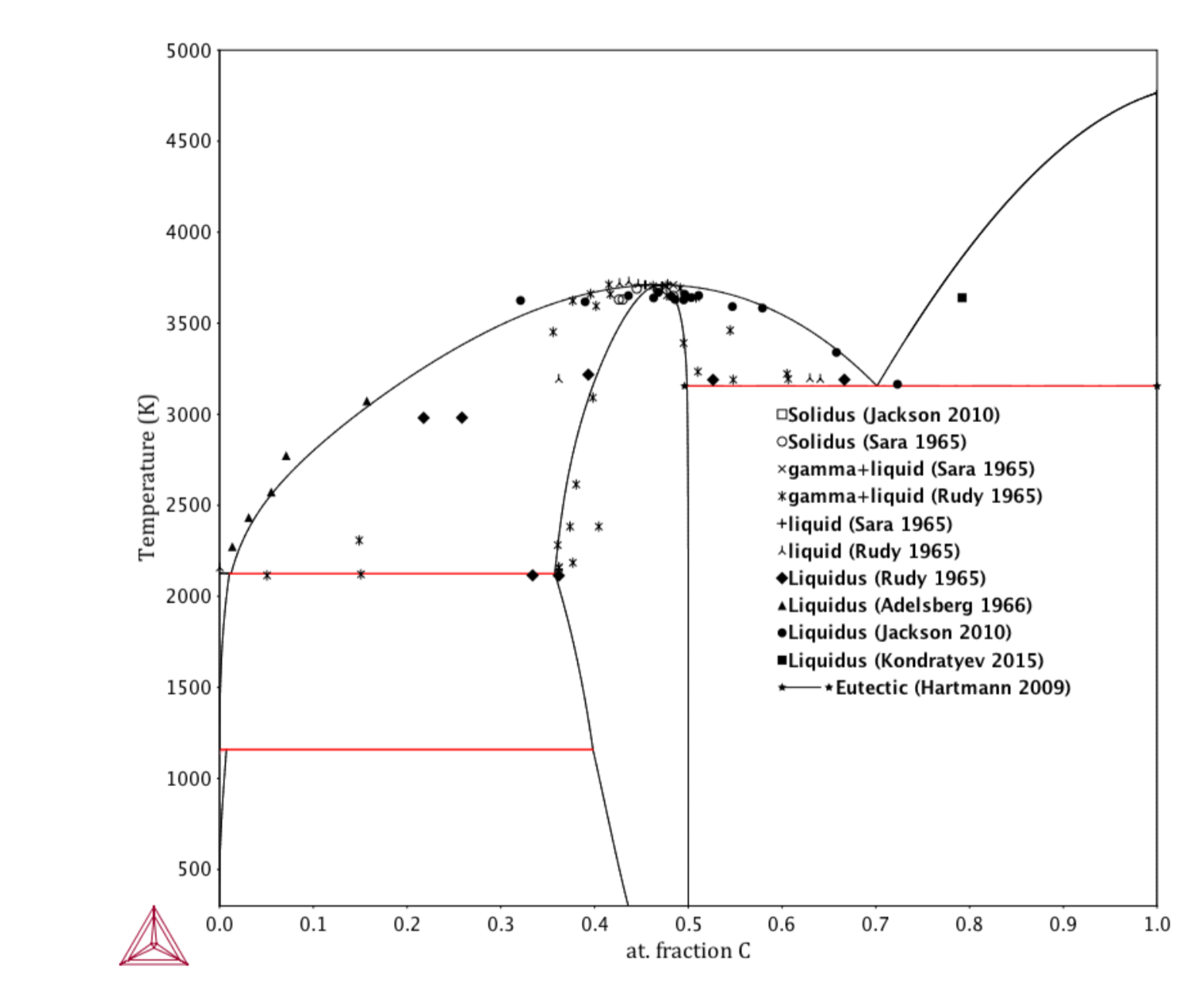
Reassessment of the C-Zr phase diagram
The carbon-zirconium phase diagram has previously been assessed using the CALPHAD approach in 1995 by Fernandez Guillermet [2]. Since then, developments in experimental techniques and theoretical methods have provided access to new information about the phase diagram and thermodynamic properties of phases within this system. In particular, fully anharmonic ab initio calculations from Duff et al. [3] were used to describe the Gibbs energy of ZrC. Other experimental insights include improved knowledge of the ZrC+C eutectic reaction, liquidus, and solidus. A review of all the data in the literature was conducted and a new CALPHAD assessment was performed incorporating the new insights [1].
- Davey T. Thermodynamic assessments of the (Zr, Hf) carbides and borides revisited and informed by the calculation of defect formation energies in ZrC. Imperial College London; 2017.
- Fernández Guillermet A. Analysis of thermochemical properties and phase stability in the zirconium-carbon system. J Alloys Compd. 1995;217:69–89.
- Duff AI, Davey T, Korbmacher D, Glensk A, Grabowski B, Neugebauer J, et al. Improved method of calculating ab initio high-temperature thermodynamic properties with application to ZrC . Phys Rev B. 2015;91(21):1–21.
